- Visibility 117 Views
- Downloads 34 Downloads
- Permissions
- DOI 10.18231/j.sajhp.2024.001
-
CrossMark
- Citation
Novel therapeutics for Hodgkin's lymphoma
Abstract
Hodgkins Lymphoma (HL) was first described by Thomas Hodgkin in 1832., is a malignant disorder, a rare B cell lymphoma (mutant lymphocytes) with reasonable outcome due to the fair cure rates achieved by modern chemotherapy and/or radiotherapy. The incidence of HL is 2.6 cases per 100,000 people, accounts for 10% lymphoma cases. The Epstein–Barr virus (EBV) is detected in nearly 45% of HL patients Most of the affected patients are between ages 20 to 40 years. HL is uncommon in children < 5 years of age. The Reed-Sternberg (RS), large cells 50 micrometers in diameter which secrete cytokines to recruit reactive cells that include IL-5 and transforming growth factor-beta (TGF-beta). The RS cells are positive for CD 30 and CD15 & sometimes for CD 20. They are negative for CD 45. The 5-year overall survival (OS) in stage 1 or stage IIa is approximately 90%;, the stage 4 disease has a 5-year OS of approximately 60%. More than 80 percent of all patients diagnosed with Hodgkin lymphoma can be cured by current treatment approaches. The cure rate is higher, approaching 90 percent, in younger patients and those with early-stage Hodgkins lymphoma (ESHL). Pragmatic therapeutic approach includes brief chemotherapy (ABVD- Adriamycin), bleomycin, vinblastine sulfate, and dacarbazine. For 3–4 cycles), For advanced-stage classic (CD30-positive) Hodgkin lymphoma, the combination of doxorubicin, vinblastine, dacarbazine, and brentuximab has emerged as a more effective, Patients with recurrent Hodgkin lymphoma would require high-dose chemotherapy followed by ASCT. Hodgkin lymphoma that recurs after ASCT would be managed by the checkpoint inhibitors nivolumab and pembrolizumab. Radiotherapy plays a role as a single modality in early stage lymphocyte-predominant HL. The role of autologous transplant was established decades ago by two randomized controlled trials demonstrating an improvement in PFS but not OS in patients with relapsed/refractory HL.
Introduction
Hodgkin’s lymphoma (HL) is a common, malignant hematological tumor of the lymph nodes (LN) and lymphatic system, accounting for approximately 10% of all lymphomas. HL comprises 2 main subtypes: (1) classical HL (cHL) and (2) nodular lymphocyte predominant HL.
There are no clearly defined risk factors for the development of this disease and the cause of HL remains unknown. Factors shown to be associated with HL include familial factors, viral exposures, and immune suppression.
Epstein-Barr virus is a risk factor for the development HL, upto 40 % cases are associated with infection. Epidemiologic and serologic studies have implicated Epstein–Barr virus (EBV) in the etiology of HL and the EBV genome was been detected in tumor specimens from patients with HL. There is also an association with human immunodeficiency (HIV) infection, in that HIV-infected patients have a significantly increased risk of HL when compared to the general population.
The JAK/STAT signaling pathway is also critically involved in Typical HL pathogenesis. Epstein-Barr virus nuclear antigen-1 (EBNA1) is also positive in affected cells.[1], [2]
|
Features |
Non Hodgkins Lymphoma |
Hodgkins Lymphoma |
|
Median Age |
65 – 70 Years |
2 peaks : 20-30 years , 50- 70 years |
|
Lymph node Enlargement |
Peripheral Lymph nodes are enlarged |
Cervical & Mediastinal Lymph nodes are enlarged |
|
Epitrochlear Lymph node |
Commonly involved |
Rarely involved |
|
Extra nodal involvement |
More Common |
Less Common |
|
Systemic or B symptoms |
Less Observed |
Commonly seen |
|
Pruritus |
Less common |
Common |
|
Alcohol induced discomfort at Lymph node site |
Absent |
Present |
|
Bone Marrow involvement |
Late |
Early |
|
Histology |
No Reed Sternberg cells (CD25 and CD71, are observed |
Reed Sternberg Cells seen - NF-kappa B, I-kappa B-alpha or c-Rel amplifications observed . CD95 gene mutations are also seen. (CD15+, CD30+, CD20-, CD45-) |
|
Symptom |
Presentation |
|
Persistent Fatigue |
Feeling of tiredness for long time |
|
Fever |
High grade persistent fever |
|
Night Sweats |
After midnight |
|
Weight Loss |
Significant after 6 months |
|
Pruritis |
More after bath or Alcoholic beverages |
|
Abdominal and Chest Pain |
Signify more extent of disease |
Patients with Hodgkin lymphoma have been shown to have high levels of antibodies against Epstein-Barr virus. HIV-1 is a human carcinogen that causes Hodgkin lymphoma via immunosuppression (indirect action). Association with tobacco smoking is controversial.[5]
|
Classical Hodgkins Lymphoma – (cHL) |
Nodular lymphocyte-predominant Hodgkin lymphoma |
|
1. Nodular sclerosis- 80 % people |
They have popcorn cells” or “LP cells” that have a marker called CD20 on their surface. |
|
2. Mixed cellularity Hodgkin lymphoma. – They have large RS Cells |
Good Prognosis |
|
3. Lymphocyte-rich |
|
|
4. Lymphocyte-depleted Hodgkin lymphoma – 1 % population |
|
|
10 - 30% of Hodgkin lymphomas come back (recur). Further treatment after recurrence is often successful. |
|
|
1. |
Mediastinal Lymph node in Chest more than 10cm |
|
2. |
Anemia |
|
3. |
High ESR |
|
4. |
Age above 60 years |
|
5. |
Low levels of Albumin and B2 microglobulin |
|
6. |
IL 10 IL 6 in Blood |
|
7. |
Bone involvement |
|
8. |
Failure to First & Second Line Chemotherapy or ASCT |
|
1. |
Histologically, it is characterized by a minority of neoplastic cells, Reed-Sternberg cells ( Bilobed central nucleus ) with lymphoid follicular hyperplasia= The initial diagnosis of HL can only be made by a biopsy. |
|
2. |
Decreased CD 4 to CD8 ratio |
|
3 |
Chest X ray for Mediastinal mass |
|
4. |
CT scan : Chest , abdomen & pelvis |
|
5. |
FDG – PET: CT, is its ability to detect metabolic changes in the areas involved with malignant lymphoma |
|
6. |
Gallium scintigraphy was used to define disease extent and response in Hodgkin’s and other lymphomas |
|
7. |
Bone marrow biopsy should be reserved for patients with stage III and stage IV Hodgkin’s lymphoma. |
Timeline history of FDA Approvals in Hodgkin lymphoma

(Adapted Fig from : Hazane Leroyer, E.; Ziegler, C.; Moulin, C.; Campidelli, A.; Jacquet, C.; Rubio, M.T.; Feugier, P.; Pagliuca, S. Filling the Gap: The Immune Therapeutic Armamentarium for Relapsed/Refractory Hodgkin Lymphoma. J. Clin. Med. 2022, 11, 6574. https://doi.org/10.3390/ jcm11216574)
Chemotherapy of Hodgkins Lymphoma
First Line Chemotherapy for Classical HD
ABVD: Doxorubicin, bleomycin , vinblastine and dacarbazine. ABVD chemotherapy is usually given every 2 weeks for 2 to 8 months.
AAVD: This regimen is similar to ABVD, but brentuximab vedotin replaces bleomycin. AAVD is given every 2 weeks for 6 months.
BEACOPP: Bleomycin, etoposide, doxorubicin, cyclophosphamide vincristine procarbazine and prednisone. There are several different treatment schedules, but different drugs are usually given every 2 to 3 weeks.
Second Line Chemotherapy or reserve regimens
ICE: Ifosfamide , carboplatin, and etoposide. ICE is usually given every 2 or 3 weeks for 2 to 3 cycles.
Brentuximab vedotin It is usually given every 3 weeks for up to 16 cycles
GVD: GVD is gemcitabine, vinorelbine (Navelbine, and doxorubicin.
ESHAP is etoposide, methylprednisolone, high-dose cytarabine, and cisplatin.
DHAP is dexamethasone, high-dose cytarabine, and cisplatin.
Other Therapies: Immunotherapy: Nivolumab and pembrolizumab, Radiation therapy - gold standard for external beam RT is 3D-CRT that use a linear accelerator (LINAC). Involved-site radiation therapy (ISRT) is sometimes preferred treat HL. It selectively treats the lymph nodes where the cancer started. External beam radiation therapy is provided 15 to 20 minutes and is typically administered five days a week for several weeks. Autologous transplantation of stem-cells is a therapeutic option for Hodgkin's lymphoma patients after the first relapse. Promising results were observed in patients with a low tumor burden at transplant.[8], [9]
|
Drug |
Mode of action |
Dose & ADR |
Indication |
|
Vinorelbine |
mitotic microtubules during the G2 and M phases are inhibited |
4 courses of 30 mg/m2 weekly are administered. Neutropenia and neuropathy are side effects |
Relapsed or refractory HD treated with a combination of vinorelbine and ifosfamide |
|
Idarubicin |
Idarubicin has antimitotic and cytotoxic activity |
idarubicin (8 mg/m2 dl, ) is administered together with etoposide and dexamethasone Myelosuppression and Leukopenia are major ADR |
Patients with relapsed or refractory HD. |
|
Bendamustine |
bendamustine causes intra- and inter-strand crosslinks between DNA bases resulting in cell death. |
70-90 mg/m2 (range 70-100 mg/m2 ) IV for one dose on days 1 and 2. Diarrhoea , Dyspepsia are major side effects |
Objective response rate of bendamustine was found to be 74.3% in relapsed/refractory HL |
|
Gemcitabine |
Interferes with DNA synthesis and targeting ribonucleotide reductase. |
Administered in a weekly schedule at a dosage of 1000 mg/m2. nausea and vomiting occur in only 20% and alopecia in only 0.5%. |
Gemcitabine in relapsed or refractory HL produces overall response rate of about 40%. |
Biologicals for Hodgkins Disease
Brentuximab vedotin is composed of 3 parts: a chimeric human-murine IgG1 that selectively targets CD30, monomethyl auristatin E (MMAE), which is a microtubule-disrupting agent, and a protease-susceptible linker that links the antibody and MMAE. Brentuximab vedotin causes apoptosis of tumor cells by preventing cell cycle progression of the G2 to M phase.
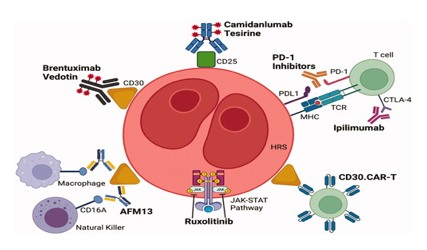
Brentuximab vedotin having half life between 4- 6 days, is indicated in adult patients for the treatment of previously untreated stage III or IV classical Hodgkin's lymphoma (cHL) in combination with doxorubicin, vinblastine, and dacarbazine. Frontline treatment with the combination of brentuximab vedotin, nivolumab, doxorubicin, and dacarbazine led to an overall response rate ORR of 98%. The most severe toxic reaction observed is progressive multifocal leukoencephalopathy.[11]
Nivolumab The ligands PD-L1 and PD-L2 bind to the PD-1 receptor on T-cells, inhibiting the action of these cells. Tumor cells express PD-L1 and PD-L2. Nivolumab, Pembrolizumab binds to PD-1, preventing PD-L1 and PD-L2 from inhibiting the action of T-cells, restoring a patient's tumor-specific T-cell response.
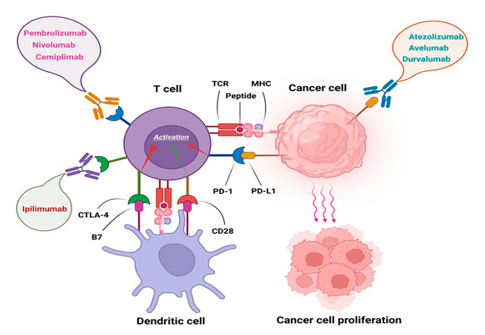
Nivolumab, an anti-PD-1 monoclonal antibody, demonstrated durable responses and manageable toxicity in a significant population of HL patients who fail both ASCT and brentuximab vedotin.
Brentuximab vedotin plus nivolumab was highly active post-autologous HSCT consolidation for patients with high-risk relapsed or refractory classic Hodgkin lymphoma, most of whom had previous exposure to either brentuximab vedotin or PD-1 blockade.[12]
Pembrolizumab combination with Brentuximab is a highly effective and safe bridge treatment to ASCT for high-risk, heavily pretreated R/R HL patients. [13]
Everolimus is a mTOR inhibitor that binds with high affinity to the FK506 binding protein-12 (FKBP-12), thereby forming a drug complex that inhibits the activation of mTOR. This inhibition reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest.
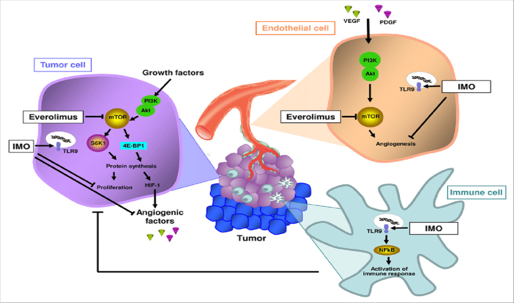
Everolimus was found to provide a response in a group of patients with refractory or relapsed Hodgkin's lymphoma who had adequate tolerability to the drug.[14]
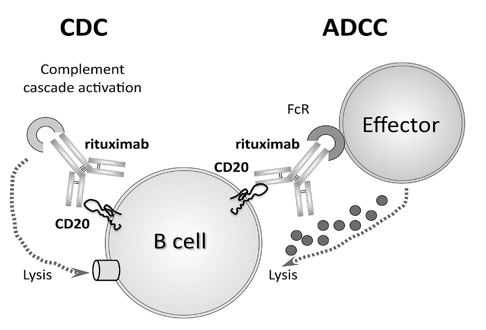
Rituximab: binds specifically to the CD20 antigen located on pre-B and mature B lymphocytes1 Because they lack CD20, stem cells and plasma cells are not selectively targeted by Rituximab. rituximab is often used to treat variant of Hodgkin lymphoma called 'nodular lymphocyte-predominant Hodgkin lymphoma' (NLPHL).[15]
Bortezomib (inhibits nuclear factor kappa B (NF-κB) with ifosfamide and vinorelbine are evaluated in childhood Hodgkins disease.
Panobinostat, Mocetinostat , Entinostat are oral nonhydroxamate HDAC inhibitors that selectively inhibits Histone deacetylases 1, 2, 3 used in refractory HL. In some cases.
Panobinostat as a single agent produced an overall response rate of 27% in patients with relapsed or recurrent Hodgkin lymphoma after stem cell transplantation.[16], [17], [18]
Lucatumumab, a human anti-CD40 mAb, was shown to cause more B-cell lysis than rituximab, by targeting CD4O. Galiximab, a primatized immunoglobulin G 1 monoclonal antibody against CD80, has high affinity binding for CD80 and induces antibody-dependent cytotoxicity (ADCC}. Mogamulizumab selectively binds to and inhibits the activity of CCR4, which may block CCR4-mediated signal transduction pathways, is being evaluated for refractory HL.
Conclusion
The major cause of HL is change in make up of DNA of white blood cells. called B lymphocytes. There is B Cell downregulation. 80%–90% of HL patients achieve permanent remission and can be considered cured. Hodgkin lymphoma has high cure either by chemotherapy or chemoradiation. Addition of immunotherapies to established chemotherapy results in improved outcomes in terms of toxicity. Chimeric antigen receptor (CAR) T-cell therapy has provided a curative option for patients with relapsed or refractory lymphoma. CD30 is an appropriate target for CAR-T-cell therapy of patients with HL. CD19 and CD123 CAR T cells that target the immunosuppressive tumor microenvironment in HL have also been investigated. Secondary malignancies such as solid tumors (lung, breast), cardiovascular disease, hypothyroidism, and fertility issues are late effects in long-term complications of HL. Vinorelbine, Idarubicin, Gemcitabine and Immunotherapy have been evaluated in resistant cases. The disease is aggressive in older patients with poor response rate. The 5 year relative survival rate is 89 %., compared to 74% of Non Hodgkins lymphoma. High-dose chemotherapy and autologous stem-cell transplant (HDC/ASCT) is standard treatment for chemo sensitive relapsed classical Hodgkin lymphoma. The current standard of care for early stage HL is two cycles of ABVD and 20 Gy radiotherapy for favourable prognosis, and four cycles of ABVD and 30 Gy for unfavourable prognosis. The standard of care for advanced HL in patients aged 16 to 60 is either 6-8 cycles of ABVD or six cycles of eBEACOPP.
Source of Funding
None.
Conflict of Interest
None.
References
- Bibas M, Antinoriebv A. EBV and HIV-Related Lymphoma. Mediterr J Hematol Infect Dis. 2009;1(2). [Google Scholar] [Crossref]
- JC, Cozen W, Steidl C, Carbone A. Hodgkin lymphoma. Nat Rev Dis Primers. 2021;6(1). [Google Scholar]
- Jarrett RF. . Viruses and Hodgkin's lymphoma. ;1:23-32. [Google Scholar]
- Vrzalikova K, Pugh M, Mundo L, Murray P. The contribution of ebv to the pathogenesis of classical hodgkin lymphoma. Ann Lymphoma. 2021;5:30-30. [Google Scholar]
- Weber 1 AL, Rahemtullah A, Ferry JA. Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am. 2003;13(3):371-92. [Google Scholar]
- Cuccaro A. Prognostic Factors in Hodgkin Lymphoma. Mediterr J Hematol Infect Dis. 2014;6(1). [Google Scholar]
- Fraga M, Forteza J. Diagnosis of Hodgkin’s disease: an update on histopathological and immunophenotypical features. Histol Histopathol. 2007;22:923-35. [Google Scholar]
- Seam P, Janik J, Longo D, Devita V. The Role of Chemotherapy in Hodgkin’s Lymphoma. Cancer J. 2009;15(2):150-4. [Google Scholar]
- Cortez A. Autologous hematopoietic stem cell transplantation in classical Hodgkin's lymphoma. Rev Bras Hematol Hemoter. 2011;33(1):10-4. [Google Scholar]
- Borchmann P, Schnell R, Diehl V, Engert A. New drugs in the treatment of Hodgkin's disease. Ann Oncol. 1998;9(5):1-3. [Google Scholar]
- Sidaway P. Brentuximab vedotin improves outcomes. Nat Rev Clin Oncol. 2023;20:1-2. [Google Scholar]
- Danecka M, Szymczyk M, Fischer J, Dankowska A, Rybka J, Mańko J. Nivolumab for relapsed/refractory classical Hodgkin lymphoma after brentuximab vedotin failure – Polish Lymphoma Research Group real-life experience. Acta Haematol Pol. 2018;49(4):228-33. [Google Scholar]
- Massaro F. Brentuximab Vedotin and Pembrolizumab Combination in Patients with Relapsed/Refractory Hodgkin Lymphoma: A Single-Centre Retrospective Analysis. Cancers. ;2022(4). [Google Scholar]
- Rocha TMBdSd, Fortier S, Fischer, TRdC, Perini GF, Gaiolla RD, Fogliatto L. Everolimus as a single agent in refractory or relapsed Hodgkin's lymphoma: the Brazilian Named Patient Program Experience. Rev Bras Hematol Hemoter. 2017;39(3):216-22. [Google Scholar]
- Weiner G. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115-23. [Google Scholar]
- Hematology ASo. Novel therapy for Hodgkin lymphoma. Connie Lee Batlevi1 and Anas Younes. 2013. [Google Scholar]
- Yashiro O. Use of panobinostat in patients with classical Hodgkin lymphoma. Int Jr of Hematologic oncology. 2014;3(3). [Google Scholar]
- Suresh T, Lee JLX, Joshi S. New antibody approaches to lymphoma therapy. J Hematol Oncol. 2014;7. [Google Scholar]
How to Cite This Article
Vancouver
Chaudhry S, Trailokya A, Naik M. Novel therapeutics for Hodgkin's lymphoma [Internet]. South Asian J Health Prof. 2024 [cited 2025 Oct 13];7(1):1-5. Available from: https://doi.org/10.18231/j.sajhp.2024.001
APA
Chaudhry, S., Trailokya, A., Naik, M. (2024). Novel therapeutics for Hodgkin's lymphoma. South Asian J Health Prof, 7(1), 1-5. https://doi.org/10.18231/j.sajhp.2024.001
MLA
Chaudhry, Sunil, Trailokya, Abhijit, Naik, Manoj. "Novel therapeutics for Hodgkin's lymphoma." South Asian J Health Prof, vol. 7, no. 1, 2024, pp. 1-5. https://doi.org/10.18231/j.sajhp.2024.001
Chicago
Chaudhry, S., Trailokya, A., Naik, M.. "Novel therapeutics for Hodgkin's lymphoma." South Asian J Health Prof 7, no. 1 (2024): 1-5. https://doi.org/10.18231/j.sajhp.2024.001
